Constructing VLE Diagrams using Raoult’s law#
Teng-Jui Lin
Content adapted from UW CHEME 375, Chemical Engineering Computer Skills, in Spring 2021.
Python skills and numerical methods
Control Flow
ChemE applications
Vapor-liquid equilibrium (VLE) diagrams
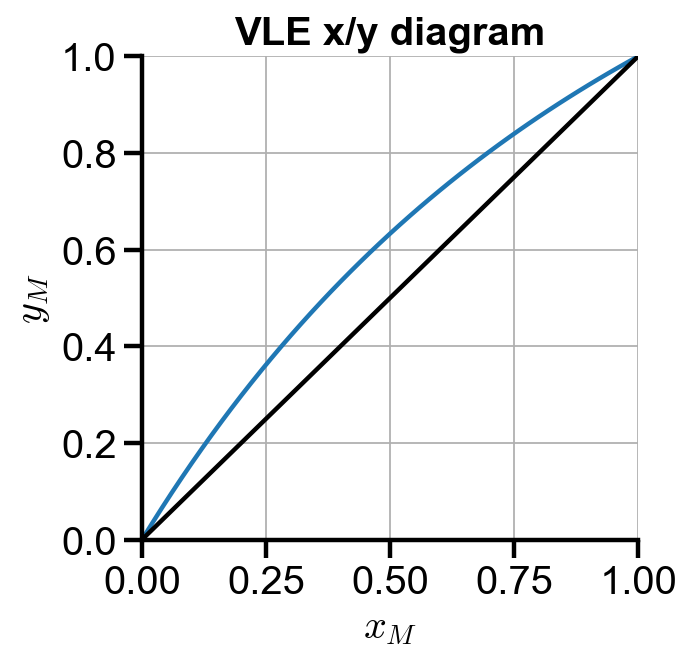
x/y diagrams
Txy diagrams
Solving binary vapor liquid equilibrium (VLE) problems#
Problem Statement. Methanol and ethanol are in binary vapor liquid equilibrium at a pressure of 1 atm. Assuming ideal gas and solution behavior, generate an x/y diagram and a Txy diagram for the methanol-ethanol system.
Solution. (Similar solution method as solving nonlinear systems.) Assuming ideal behavior, Raoult’s law states that the partial pressure of one component is equal to the vapor pressure of the component times its liquid mole fraction
where the vapor pressure can be determined by Antoine’s equation
Note that Antoine’s equation may take different form and units when using different tabulated values.
Let methanol be M and ethanol be E, we have the following equations, known, and unknown values.
Known values#
Units of values in Antoine’s equation
P [=] mmHg
T [=] deg C
Parameters of Antoine’s equation (FRB Table B.4)
Compound |
A |
B |
C |
Valid T Range |
|---|---|---|---|---|
Methanol (M) |
8.08097 |
1582.271 |
239.726 |
14.9 - 83.7 |
Ethanol (E) |
8.11220 |
1592.864 |
226.184 |
19.6 - 93.4 |
Known liquid composition
Known pressure
Unknown variables#
Composition of liquid phase:
Composition of vapor phase:
Temperature:
Governing equations#
The raw equations are:
Raoult’s law and Antoine’s equation
conservation of mass (mole, since nonreactive)
Convert all the equations so they’re in the general form of
Raoult’s law and Antoine’s equation
conservation of mass
We can now solve the four nonlinear equations with respect to the four unknown variables given the known values at each
Implementation#
In this approach, we use scipy.optimize.fsolve() to solve the nonlinear system directly at each
import numpy as np
from scipy.optimize import fsolve
import matplotlib.pyplot as plt
def system(X, params):
'''System of nonlinear equations'''
# X -> unknown variables
# params -> known values
xe, ym, ye, T = X
xm, P, Am, Bm, Cm, Ae, Be, Ce = params
# set up system of equations
# check form of Antoine's eqn (exp or 10^)
eqns = np.array([
ym*P - xm*10**(Am - Bm/(Cm + T)),
ye*P - xe*10**(Ae - Be/(Ce + T)),
1 - ym - ye,
1 - xm - xe
])
return eqns
# known values
P = 1 * 760 # atm -> mmHg
step = 0.02
xm = np.arange(0, 1+step, step)
params = [xm, P,
8.08097, 1582.271, 239.726,
8.11220, 1592.864, 226.184]
# initial guesses
X0 = [0.5, 0.5, 0.5, 100]
# initialize arrays to store iteration results
xm_len = len(xm)
xe = np.zeros(xm_len)
ym = np.zeros(xm_len)
ye = np.zeros(xm_len)
T = np.zeros(xm_len)
# iterate to solve for VLE curve
for i in range(xm_len):
params[0] = xm[i]
xe[i], ym[i], ye[i], T[i] = fsolve(system, X0, params)
# plot settings
%config InlineBackend.figure_format = 'retina'
%matplotlib inline
plt.rcParams.update({
'font.family': 'Arial', # Times New Roman, Calibri
'font.weight': 'normal',
'mathtext.fontset': 'cm',
'font.size': 18,
'lines.linewidth': 2,
'axes.linewidth': 2,
'axes.spines.top': False,
'axes.spines.right': False,
'axes.titleweight': 'bold',
'axes.titlesize': 18,
'axes.labelweight': 'bold',
'xtick.major.size': 8,
'xtick.major.width': 2,
'ytick.major.size': 8,
'ytick.major.width': 2,
'figure.dpi': 80,
'legend.framealpha': 1,
'legend.edgecolor': 'black',
'legend.fancybox': False,
'legend.fontsize': 14
})
# x/y diagram
fig, ax = plt.subplots(figsize=(4, 4))
ax.plot(xm, ym)
ax.plot([0, 1], [0, 1], color='black')
ax.set_xlabel('$x_M$')
ax.set_ylabel('$y_M$')
ax.set_title('VLE x/y diagram')
ax.set_xlim(0, 1)
ax.set_ylim(0, 1)
ax.grid(True)
# main Txy diagram
fig, ax = plt.subplots(figsize=(4, 4))
ax.plot(xm, T, color='black')
ax.plot(ym, T, color='black')
ax.set_xlabel('$x_M, y_M$')
ax.set_ylabel('$T \ [^\circ C]$')
ax.set_title('VLE Txy diagram')
ax.set_xlim(0, 1)
ymin = 63
ymax = 80
ax.set_ylim(ymin, ymax)
ax.grid(True)
# colored phase regions
ax.fill_between(xm, ymin, T, color='blue', alpha=0.1)
ax.fill_between(ym, T, ymax, color='red', alpha=0.1)
ax.text(0.12, 65, 'Liquid', color='blue')
ax.text(0.67, 77.5, 'Vapor', color='red')
ax.text(0.35, 69.2, 'Two-phase', color='black', rotation=-38, size=16)
Text(0.35, 69.2, 'Two-phase')
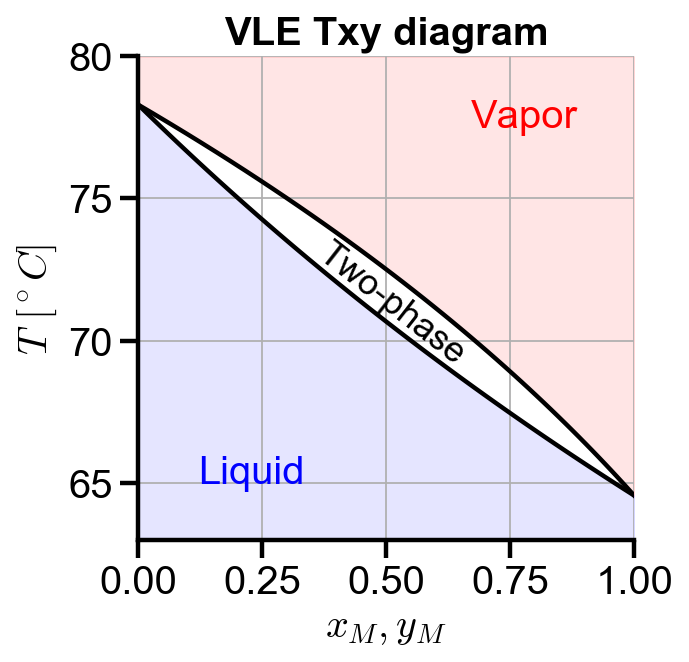
The temperature range of the Txy diagram is within the valid T range of the Antoine’s coefficients.

